Function and plasticity of transplanted human neurons: from circuit development to brain repair.
A number of prominent neurological diseases, such as stroke, trauma, and specific degenerative conditions (such as ALS) could benefit in principle from therapies enabling to replace lost or dysfunctional cortical neurons.
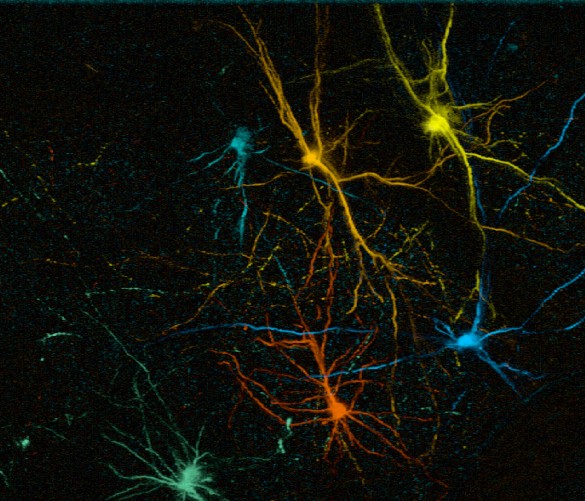
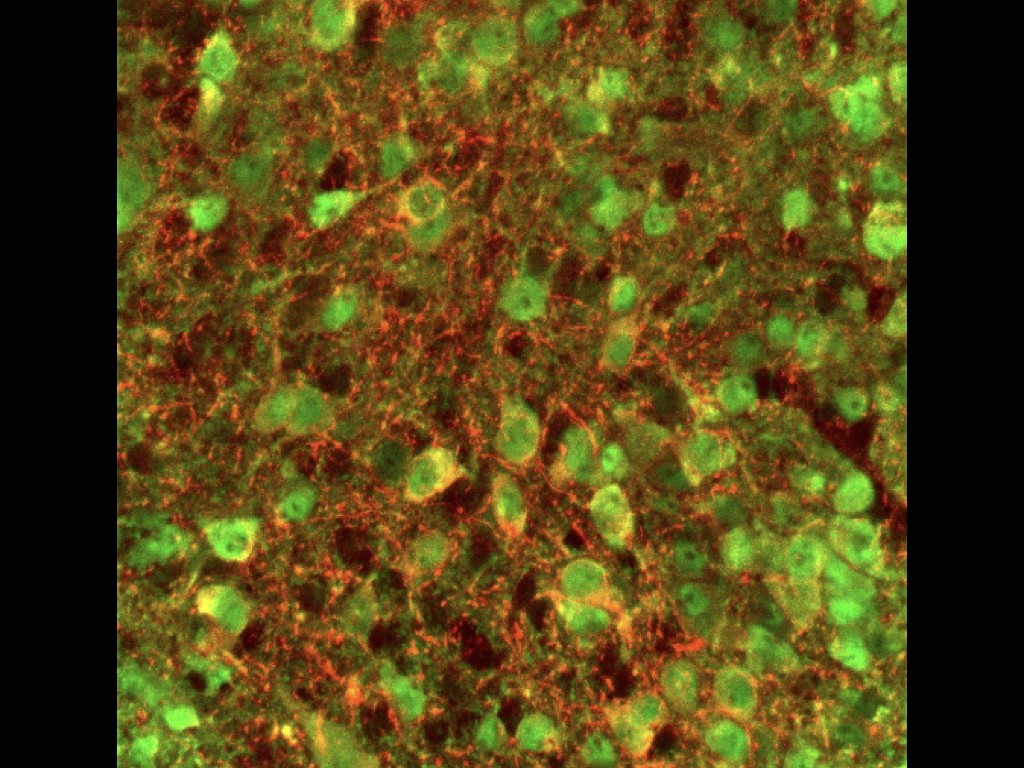
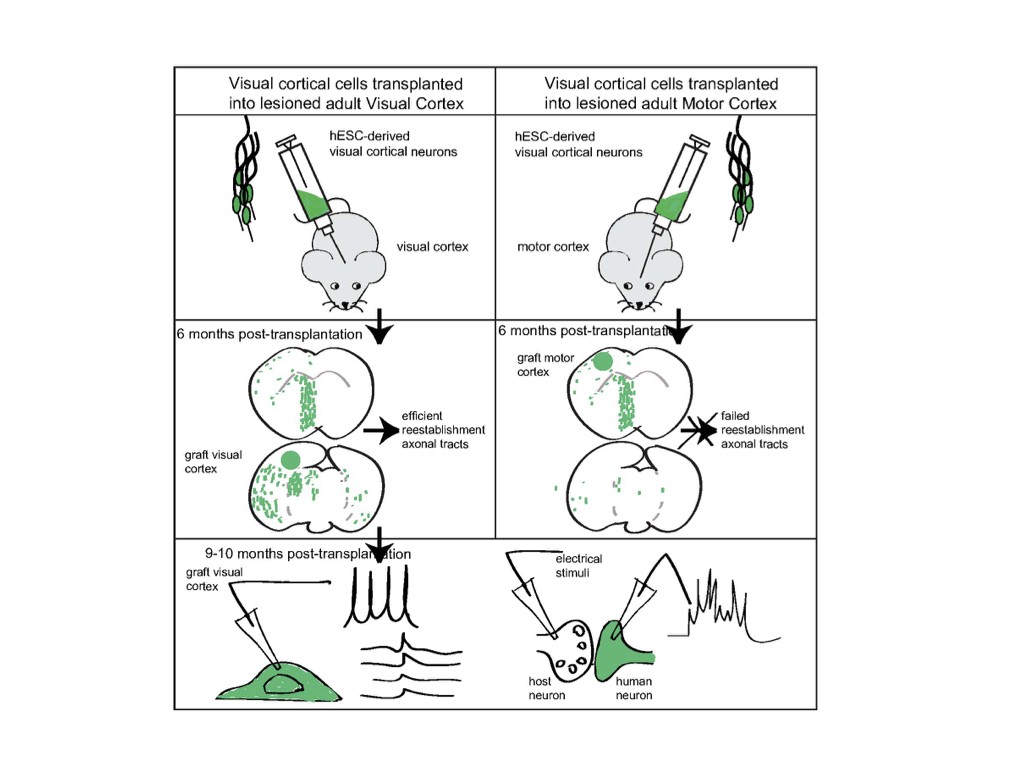
We have recently tested this directly using mouse and human ESC-derived cortical neurons in an experimental model of neurotoxic lesioning in the adult mouse cortex : we found that transplanted neurons displayed robust integration and can contribute to reestablishment of cortical projections (Michelsen Neuron 2015 ; Espuny-Camacho Cell Reports 2018).
However it remains unclear whether transplanted neurons derived from pluripotent stem cells can integrate in a complex circuit and contribute to genuine restoration of lost function. Indeed, the functional characterization of transplanted neurons in the context of brain repair, as well as their impact on host circuitry function and plasticity, appears as a major, yet unreached milestone to the road of effective and reliable cell replacement therapies of the adult brain.
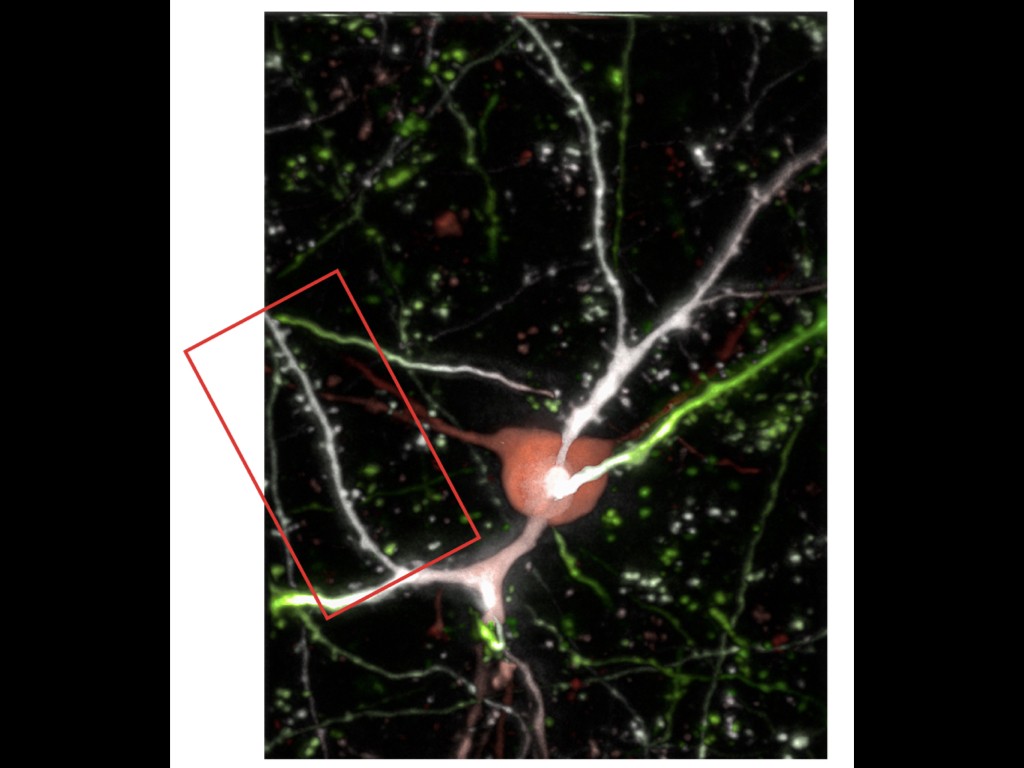
To address this key question we test whether and how the transplanted neurons display connectivity and functional properties in the host circuits that indicate physiological integration. The neurons are characterized molecularly, morphologically and functionally using single cell moprhology, electrophysiology and transcriptome analysis. Moroever thanks to a close collaboration with the Bonin Lab at the nearby NERF institute (https://www.nerf.be/research/nerf-labs/bonin-lab), we use in vivo multiphoton imaging to determine the developmental dynamics and function of the transplanted human neurons in the transplanted animals. This approach already led to the discovery that transplanted human neurons can display surprisingly precise patterns of connectivity enabling to respond to visual stimuli in a highly tuned fashion, like the mouse host neurons (Linaro et al. Neuron 2019).