Mechanisms controlling the timing of human neuronal development: human brain neoteny.
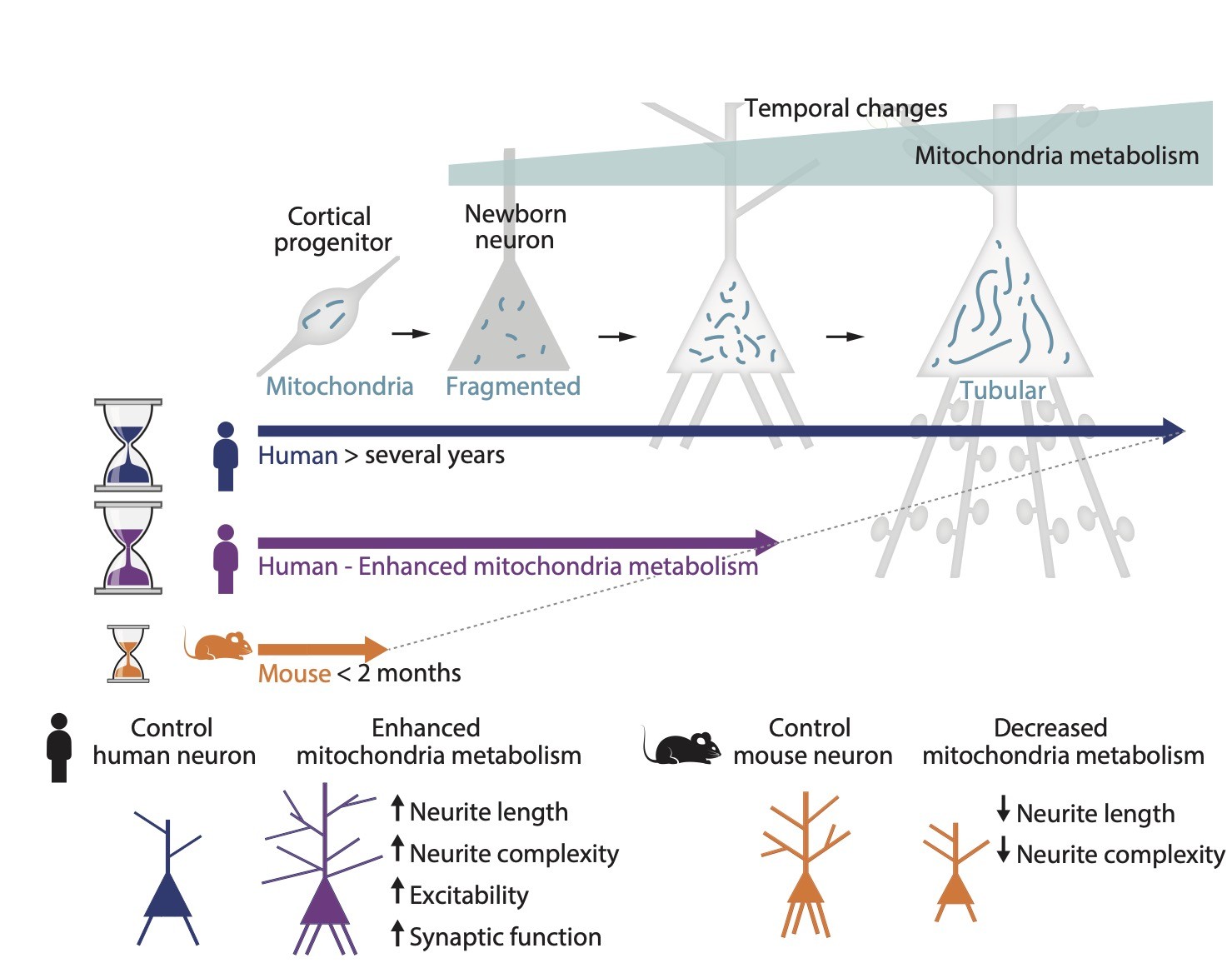
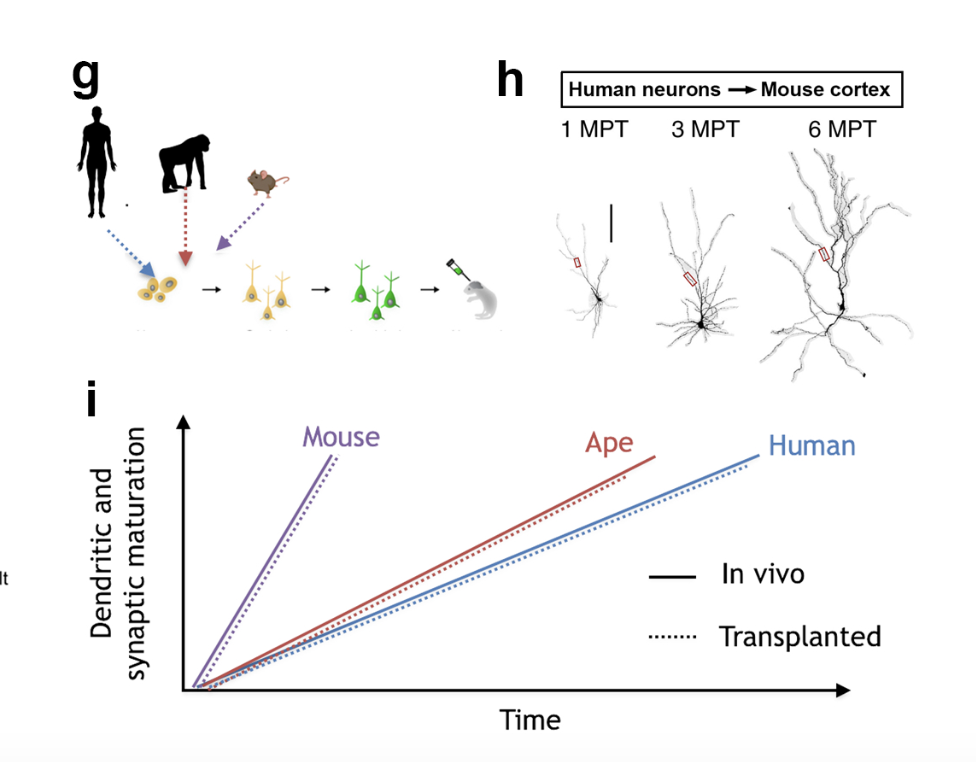
One important distinctive feature of human brain development is its unusually protracted rate of maturation, or "neoteny" (retention of juvenile features in a mature organism). Neoteny is particularly striking for human cortical neurons, that take months to years to develop, while non-human primate or rodent neurons takes only weeks (Vanderhaeghen and Polleux, Nat. Rev. Neurosci. 2023). Human brain neoteny is thought to lead to prolonged periods of neural plasticity, and thereby enhanced optimization of neural circuits in response to the environment. But very little is known about the mechanisms underlying the timing of brain maturation and neuronal differentiation, and their impact on neural circuit function.
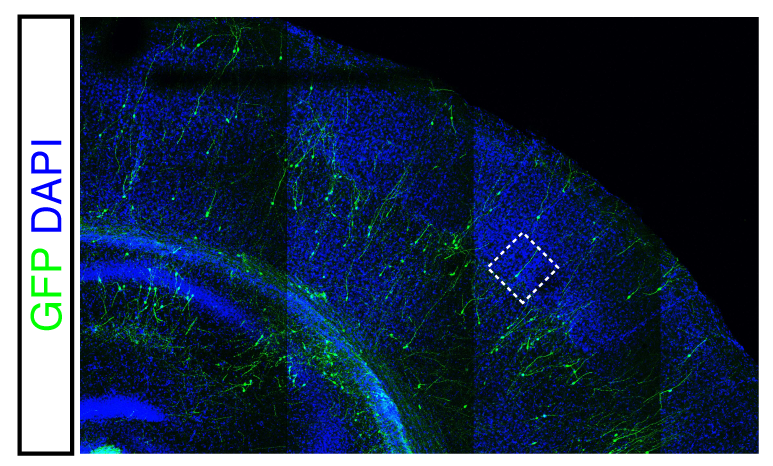
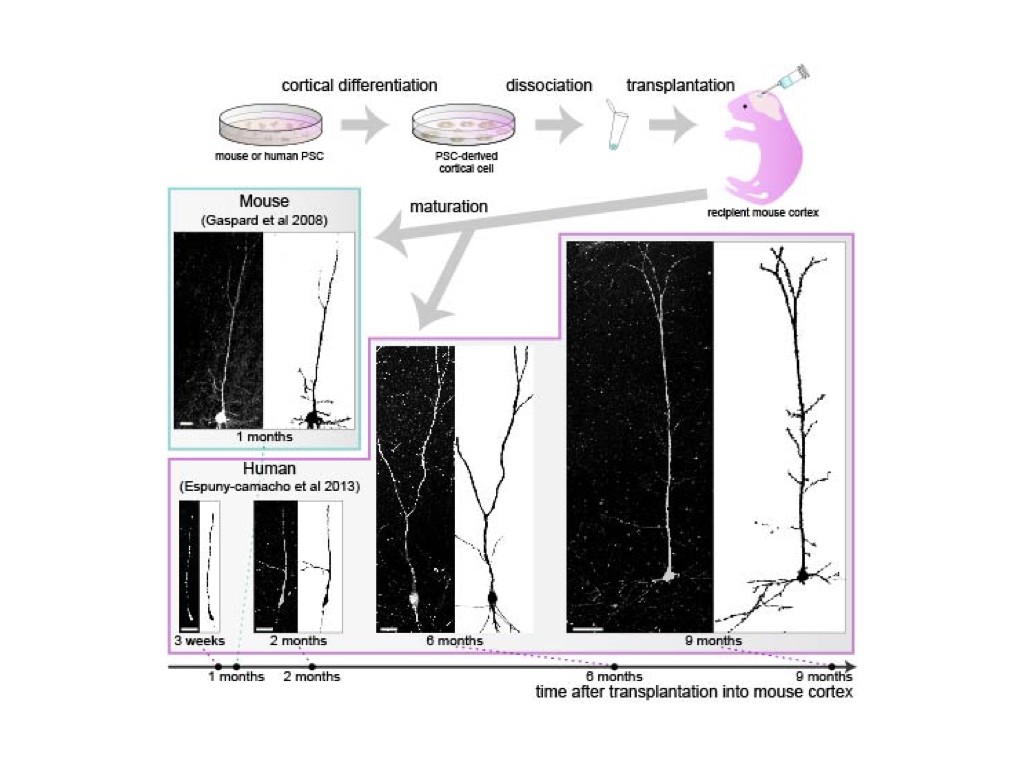
We previously found that protracted maturation of human cortical neurons can also be observed even following their transplantation into the mouse developing brain (Espuny-Camacho et al. Neuron 2013; Linaro et al. Neuron 2019), suggesting that intrinsic mechanisms play an important role in the regulation of the timing of neuronal differentiation.
We are now searching for the molecular mechanisms that control the intrinsic timing of maturation of cortical neurons.
Among these, we identified mitochondria dynamics and metabolism as key timers of species-specific neuronal development, in driect link with human neuronal neoteny (Iwata et al. Science 2020; Iwata et al. Science 2023). We are now pursuing these efforts to understand how metabolism and other global cellular mechanisms such as proteostasis and chromatin remodeling can impact neuronal developmental timing.
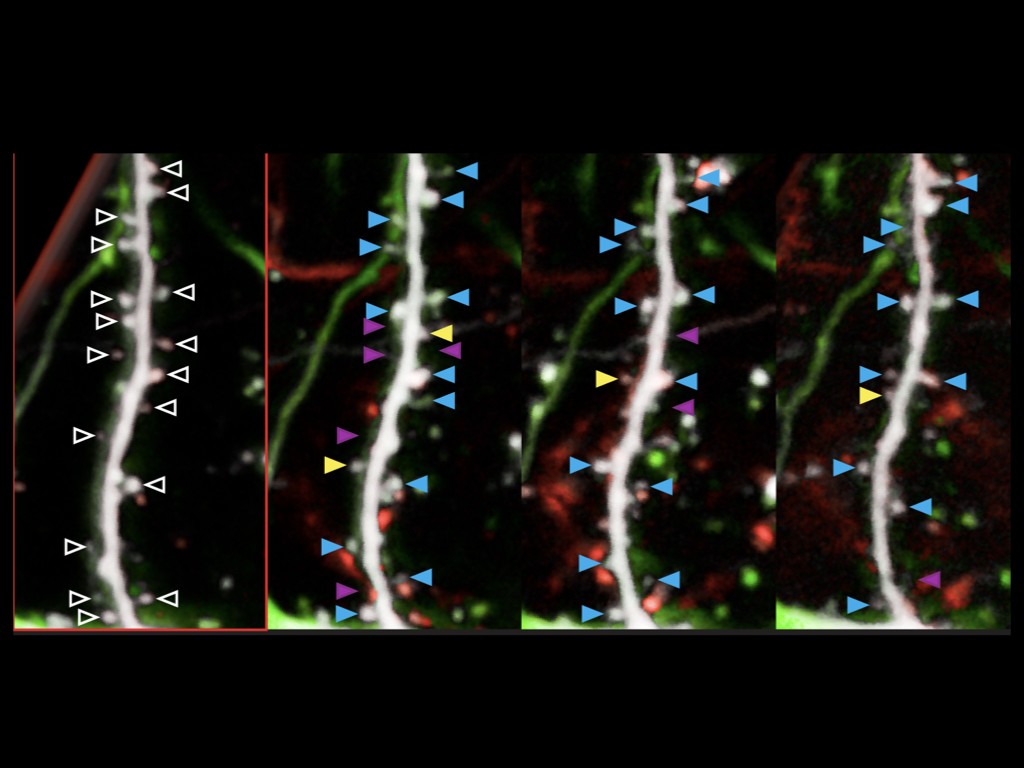
We also discovered that human-specific genes SRGAP2B/C are required for the prolonged development of human cortical neurons in vivo, by regulating the activity of SYNGAP1, an important synaptic protein implicated in intellectual deficiency and autism spectrum disroders (Libé-Philippot et al. biorxiv 2023).
In parallel, we combine comparative multi-ome analyses of developing neurons of various species (in close collaboration with the Stein lab at CBD (https://www.steinlab.org)) followed by crisp/cas9 functional screens in human neurons to identify the genetic pathways controlling the timing of cortical neuron development and connectivity.