Modeling of neurodevelopmental disorders in human neurons in vivo.
Neurodevelopmental disorders, in particular intellectual deficiency (ID) and autism spectrum disorders (ASD) constitute a major class of brain disorders with a heavy societal burden and very few therapeutic options to this day, mainly because of our lack of knowledge of the underlying mechanisms at the levels of the affected neuronal circuits.
In line with this gap of knowledge it has remained almost impossible to study experimentally human defects at the neuronal level, given the relative inaccessibility of live human neuronal material.
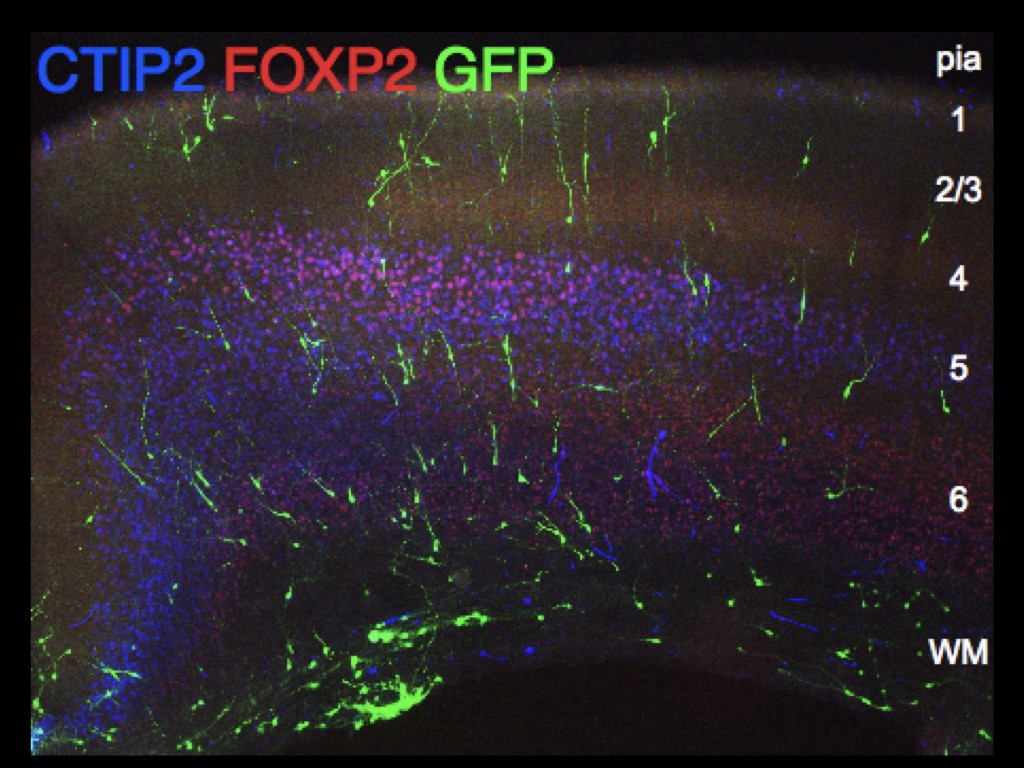
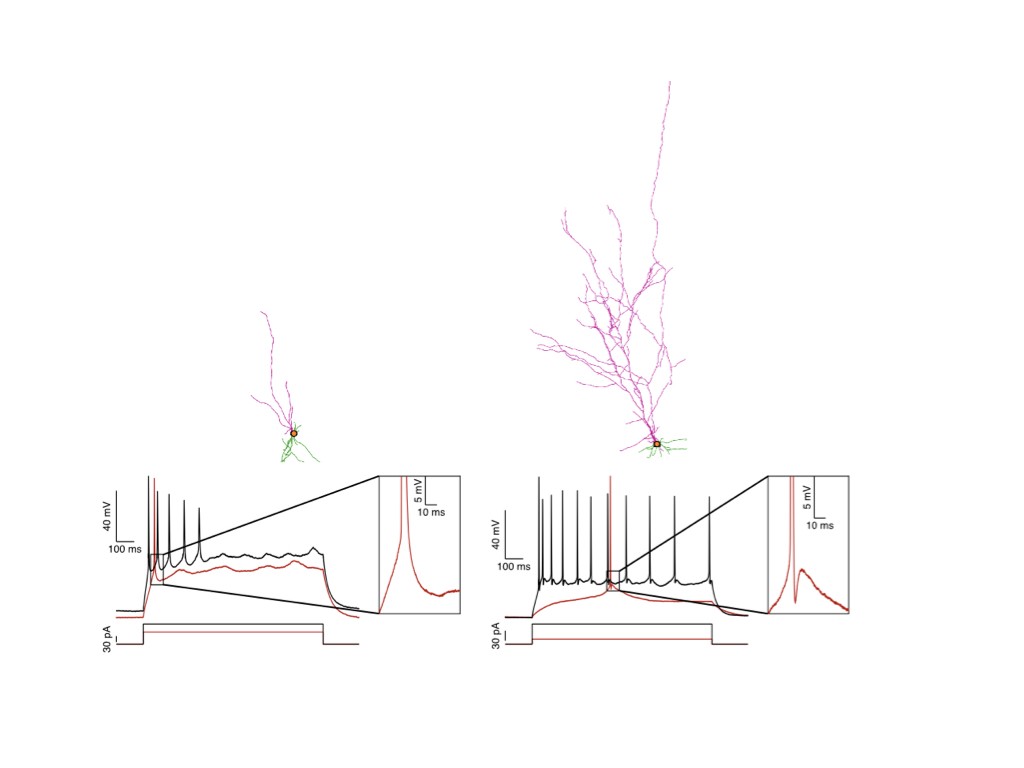
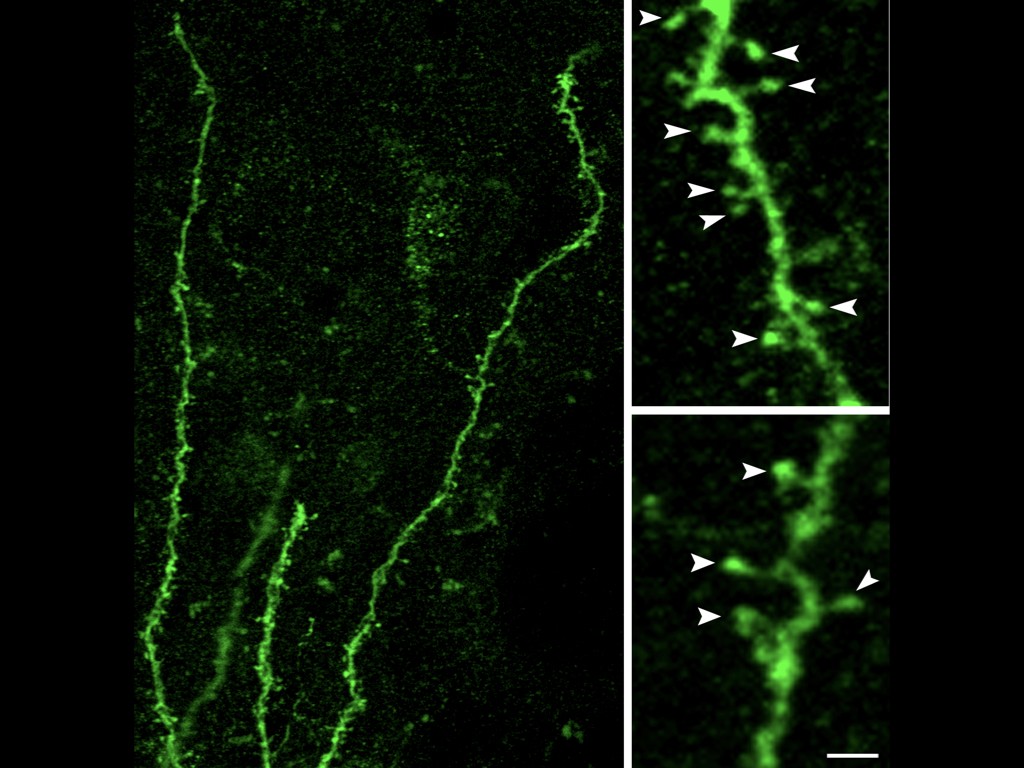
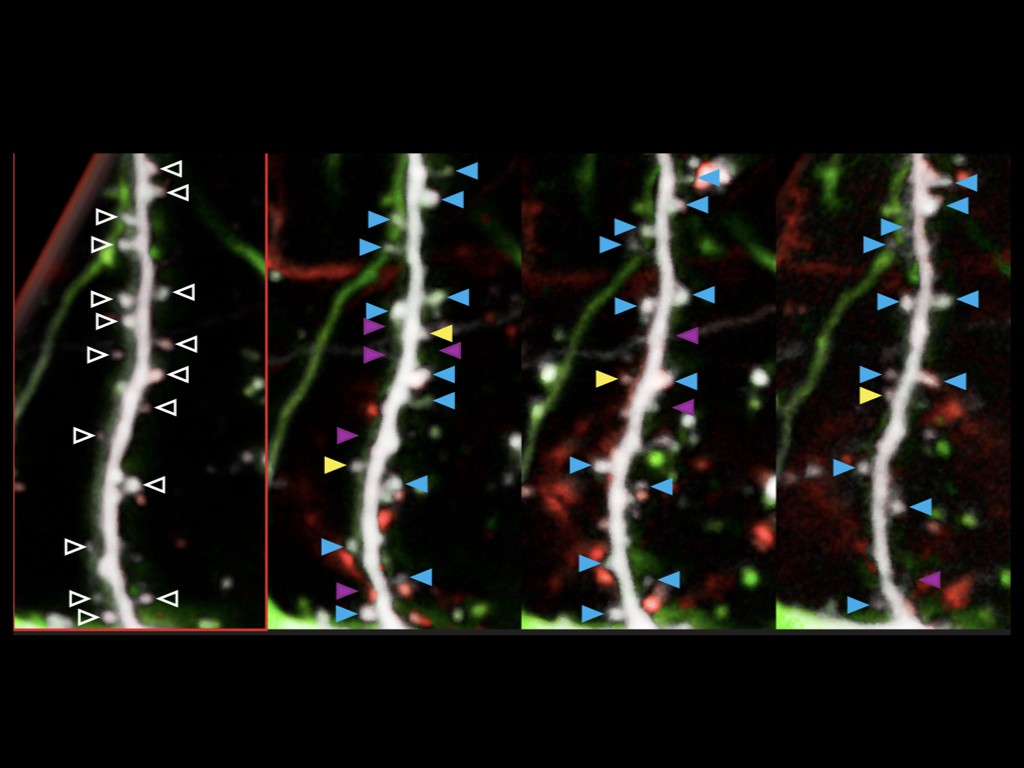
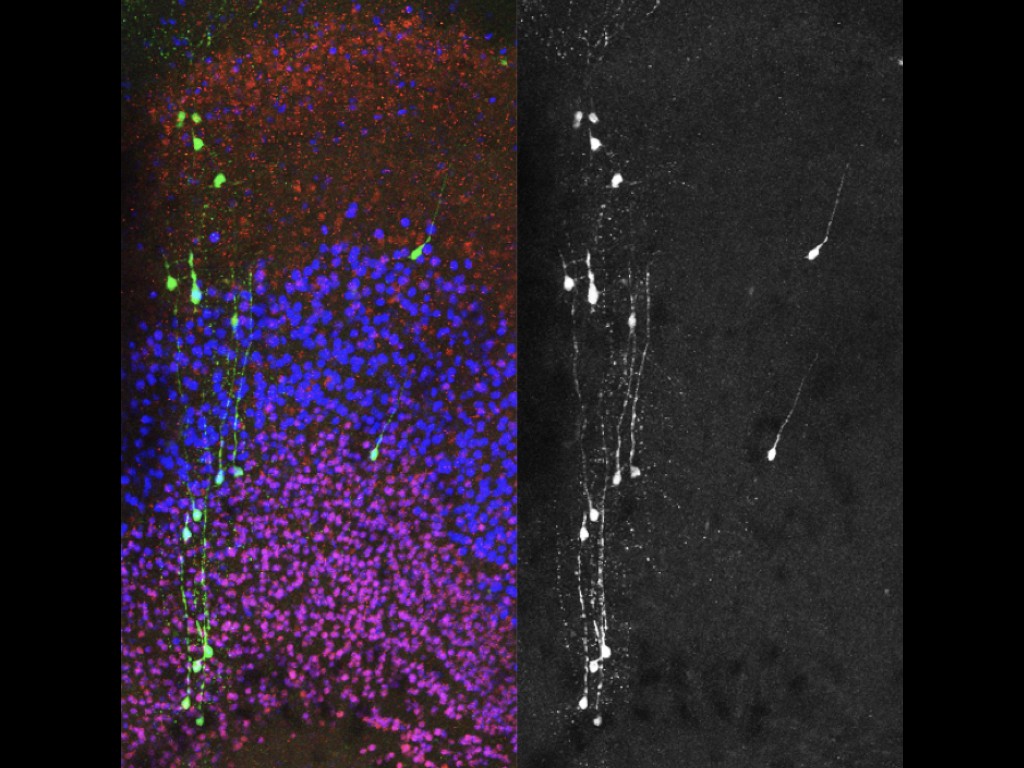
Our team has pioneered in vivo models of human cortical neuron development, whereby human pluripotent stem cells can be differentiated efficiently into pyramidal projection neurons, followed by xenotransplantation in the mouse cortex, where they display functional integration in the host neural circuits (Espuny-Camacho Neuron 2013 ; Neuron 2017 ; Cell Reports 2018). The neurons are then characterized morphologically and functionally, using combinations of single cell morphology, electrophysiology, and transcriptomics. Moreover, thanks to a close collaboration with the Bonin lab at the nearby NERF institute (https://www.nerf.be/research/nerf-labs/bonin-lab), we use in vivo multiphoton imaging to determine the developmental dynamics and function of the transplanted human neurons in awake animals.
We are now using this technology to study the formation and plasticity of cortical circuits involving human neurons affected by specific mutations leading to human neurodevelopmental disorders. The affected genes encode proteins thought to be important for the development of human synapses, as well as more general regulators of neuronal specification. The genes are first targeted by Crispr/Cas9 technology in human pluripotent stem cells or neural cells, followed by cortical differentiation in vitro and in vivo following xenotransplantation in the mouse. This approach has enabled us to reveal that the disruption of SYNGAP1, an important gene mutated in fomrs of intellectual deficiency associated with autism spectrum disorder, leads to acceleration of neuronal maturation in vivo, thus providing evidence for disrupted neoteny in a model of neurodevelopmental disorder.