Microcephaly genes / linking neurogenesis and evolution of brain size
Novel insights on human cortical development and evolution may come from diseases where species-specific developmental mechanisms have been implicated. Particularly striking among these is non-syndromic primary microcephaly (MCPH). MCPH is a neurodevelopmental disorder of genetic origin characterized by smaller brain size, particularly the cerebral cortex, while the rest of the body is essentially normal.
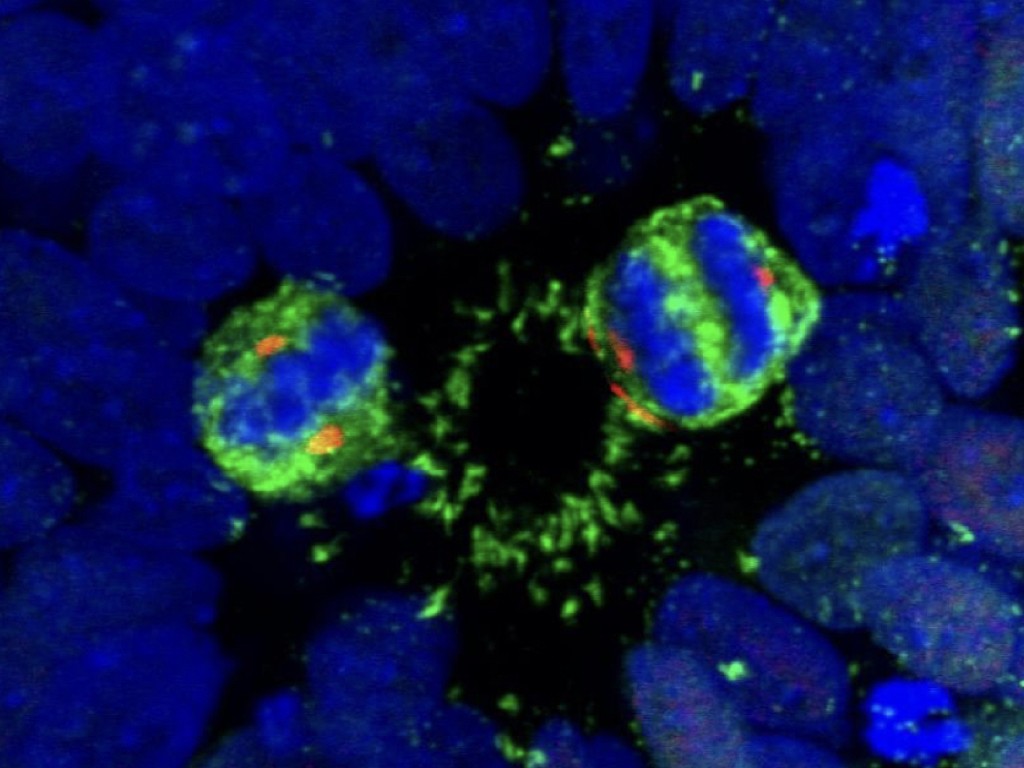
Interestingly, many MCPH genes, including the two most frequently mutated genes, ASPM and WDR62, encode proteins found at the level of the mitotic spindle and centrosomes, but the mechanisms underlying MCPH brain-specific defects remain poorly known. Morever similar mutations generated in the orthologous genes in the mouse do not lead to similarly prominent defects, suggesting that some key aspects of MCPH are linked to species-specific control of brain size and patterning.
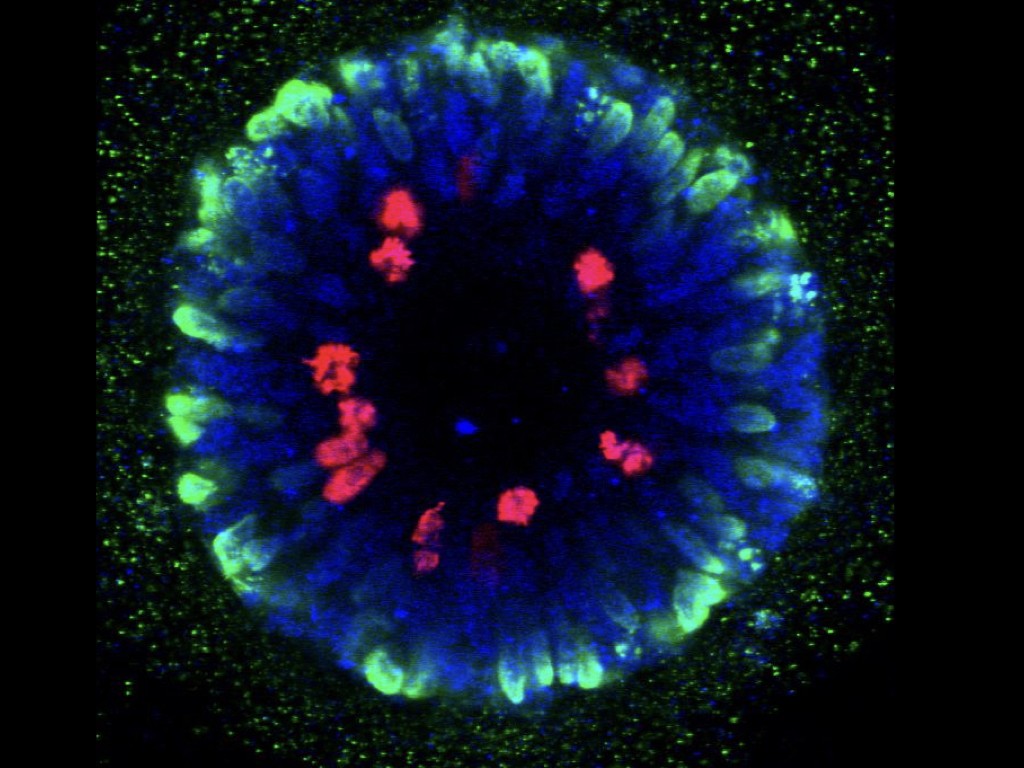
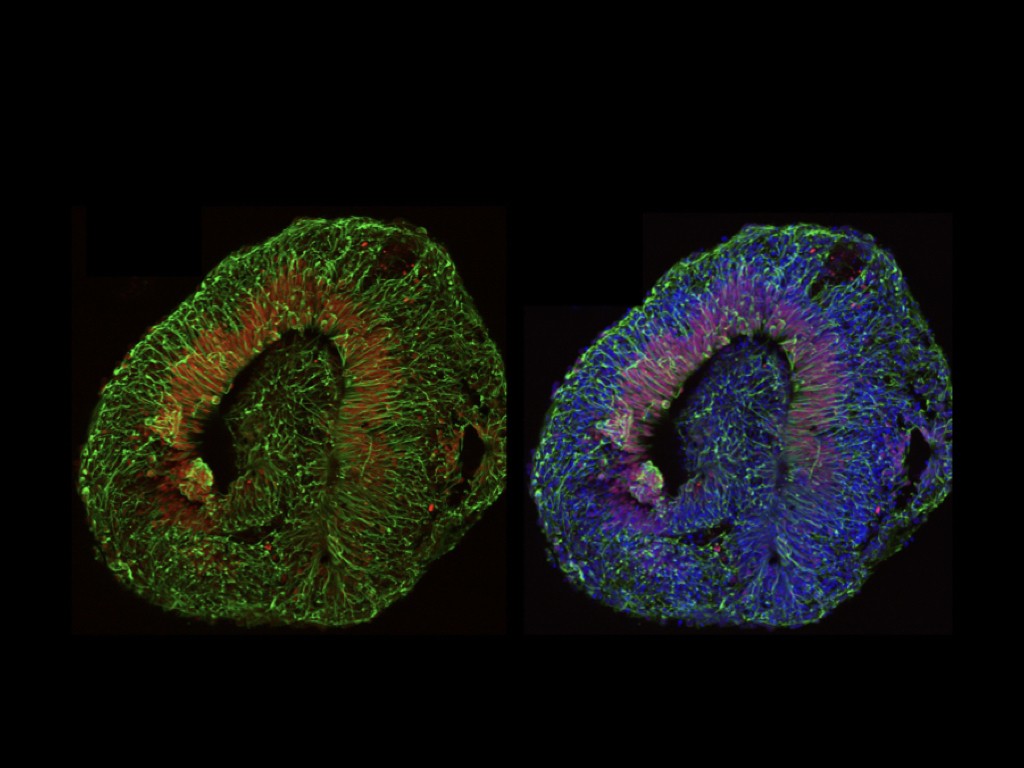
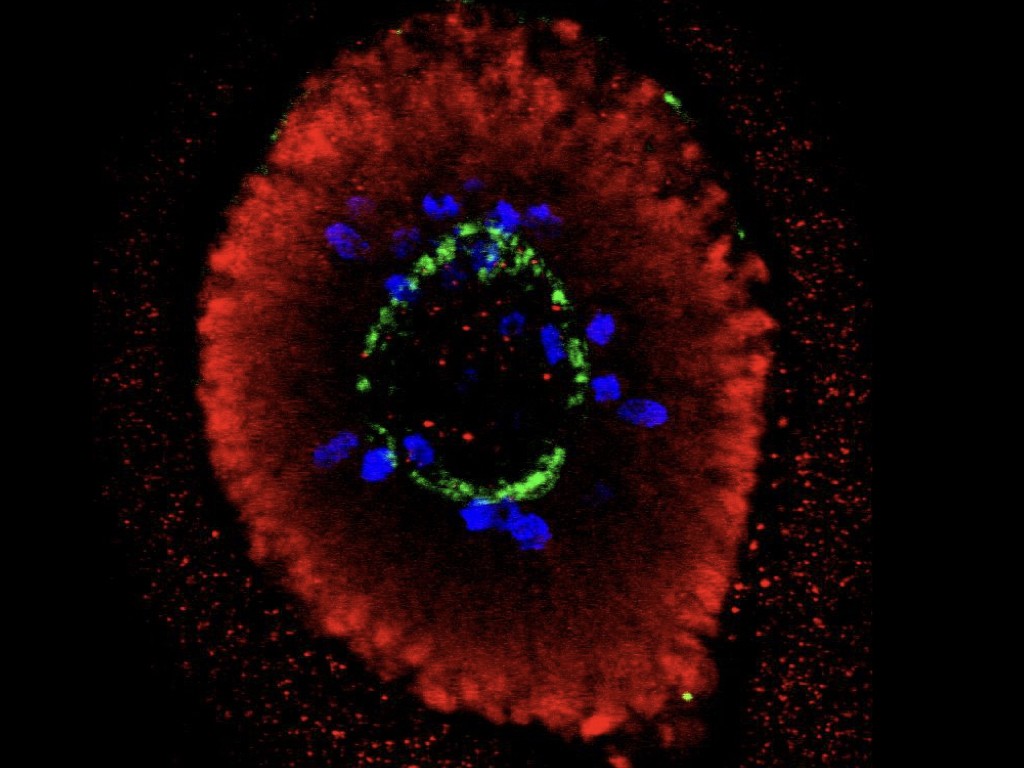
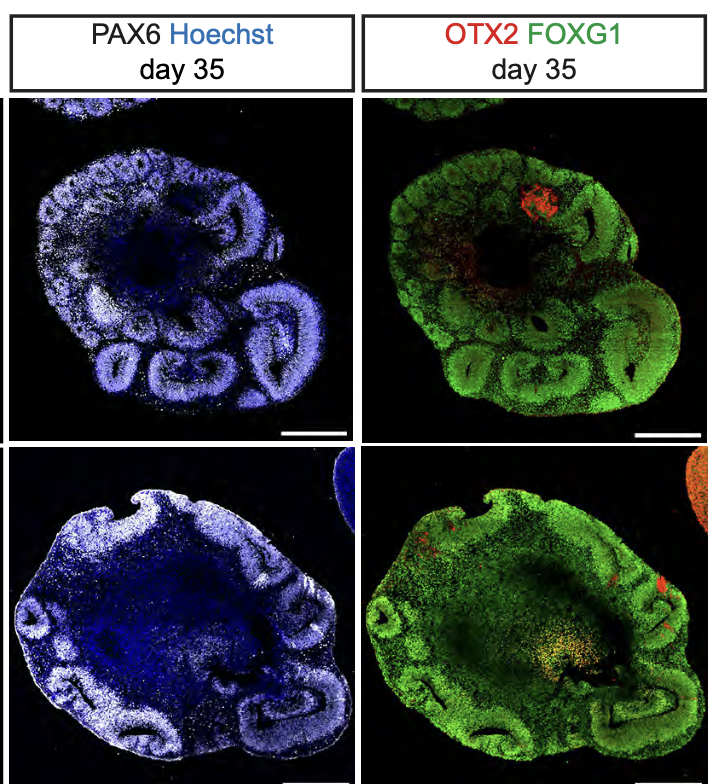
In order to understand the significance of MCPH genes in relation to human brain evolution and microcephaly, we study their function in human cortical progenitors displaying specific mutations causing MCPH. We combine technologies of induced pluripotent stem cells (iPSC) generated from patients' skin cells, together with genomic editing, and several in vitro cellular models of corticogenesis, including 2D cultures and 3D models (organoids) developed in our and other labs (Espuny-Camacho Neuron 2013; Linaro Neuron 2019; van Heurck Neuron 2023; van Benthem biorXiv 2023). This way we aim to determine what are the cellular and molecular consequences of MCPH gene mutations in human corticogenesis, in order to define the pathophysiology mechanisms of MCPH, and how they may relate with species-specific mechanisms of human cortical neurogenesis.